Thibaud THIOLLIER
A new model for the study of brain bioavailability : central nervous system microdialysis in awake macaque
octobre 2013 Directeur(s) de thèse : Erwan BEZARD Résumé de thèseWhile working on my thesis, I focused on the function of the blood-brain barrier (BBB) and its impact in drug development to target the central nervous system (CNS). Indeed, the BBB is a filter involved in CNS homeostasis but also limiting of the crossing of xenobiotics such as drugs, which constitutes an obstacle to drug development for brain diseases
Indeed, brain disorders (psychiatric, neurological and neurosurgical diseases all together) figure amongst the leading causes of disease and disability. World Health Organization data suggests that brain disorders represent 35% of all diseases in Europe. The European Brain Council estimated that over 1 quarter of Europeans are currently living with a brain disorder. The total annual cost of brain disorders in Europe was estimated to €400 billion in 2004. These costs reflect the growing needs of the European population and indirectly highlight the required efforts for developing therapeutics focusing on the brain.
However, several obstacles that slow down the process of developing new successful drugs have been identified. Among several factors, the poor brain bioavailability is acknowledged as a primary limiting factor. Despite this statement, both brain bioavailability and brain pharmacodynamic are either unknown or globally overlooked during the drug development process. A direct investigation of these parameters comes often late in the process and with only partial answers provided. Currently, 3 methods allow exploring in vivo brain pharmacokinetic: cerebral spinal fluid sampling analysis, Positron Emission Tomography imaging and brain microdialysis. Each approach has its own constraints, the first provides restricted information, the second is expensive and limited to a mainly academic use, and the third is often carried out on rodent models making the transferability of data in complex man
The research project is centred on the issue of BBB crossing by drugs and followed two axes of development. The first is focused on the study of the crossing in the particular context of Parkinson’s disease. The second addresses the lack of appropriate model used in the development of a new drug and presents a possible solution, intracerebral microdialysis in awake macaque.
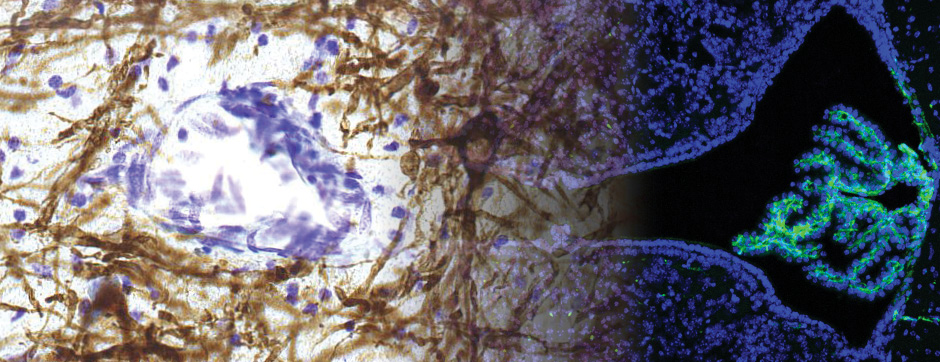
Studies linked with Parkinson’s disease have confirmed that, first it is difficult to translate data from preclinical studies to the clinic. Indeed, the trial of statins has proved ineffective in the treatment of levodopa-induced dyskinesia in parkinsonian patients. This failure can be explained in part by insufficient brain bioavailability of the active compound. Secondly, the BBB has changed on the gold standard model of Parkinson’s disease, macaques treated with 1-methyl 4-phenyl 1,2,3,6-tetrahydro pyridine (MPTP). In fact, it appears that the organic cation transporters have a lower activity in MPTP monkeys compared to the control animals.
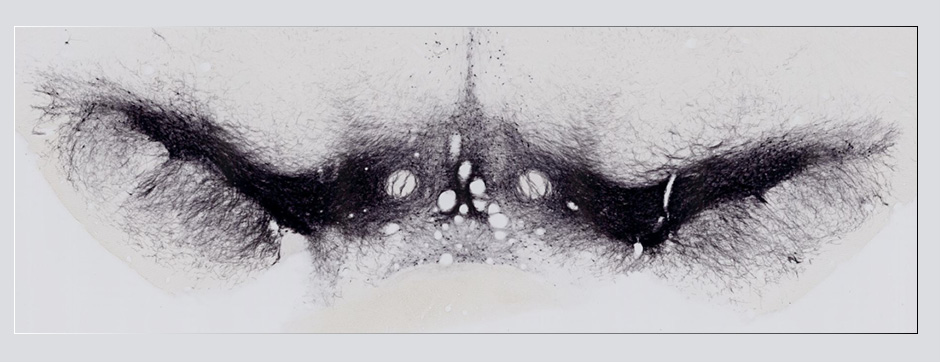
The work done along the second axis shows the feasibility of sampling brain extracellular fluid by microdialysis in awake macaque, over a period of 5 hours and in two brain areas simultaneously.