Lise Guillhemsang
Network dynamic of Basal Ganglia circuits in normal and parkinsonian conditions
décembre 2022 Directeur(s) de thèse : Thomas Boraud / Teresa Morera Herreras Résumé de thèseBasal ganglia (BG) circuits are involved in different functions from movement control to cognitive/motivational processes. The loss of dopamine (DA) in these circuits triggers Parkinson’s disease (PD) characterised by devastating motor impairments known as akinesia and bradykinesia. In addition to DA depletion, PD patients present an early serotonergic (5-HT) alteration at the level of the BG nuclei.
5-HT modulates the activity of BG circuitry by acting on a large variety of 5-HT receptor subtypes. Among them, the 5-HT2A receptor is expressed in both motor and associative/limbic territories of the BG nuclei being implicated in regulation of motor, executive and cognitive functions, as well as automatisms. In addition, 5-HT2A receptors may also play a role in diseases linked to BG dysfunction, such as PD. However, the role of the 5-HT2A receptor in cortical-BG information processing is still not well understood.
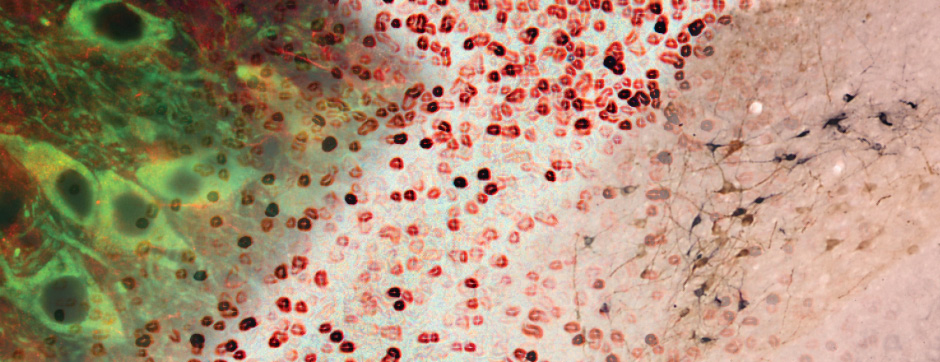
Therefore, in the first part of my PhD, we investigated the specific contribution of 5-HT2A receptors on the dynamic response of BG output nucleus to motor and medial prefrontal (mPF) cortical information. For that purpose, in vivo single-unit extracellular recordings of lateral and medial substantia nigra pars reticulata (SNr) neurons along with simultaneous electrical stimulation of the motor and mPF cortex were used to assess the effect of 5-HT2A receptor activation/blockade. The results showed a topographical-dependent dissociation in the effects triggered by the 5-HT2A agonist TCB-2, which specifically increased the medial SNr neuron activity and had preferential action on mPF cortical information processing through the striato-nigral direct pathway. These findings provide novel evidence about the specific signature of 5-HT2A receptors on the dynamic regulation of BG circuits.
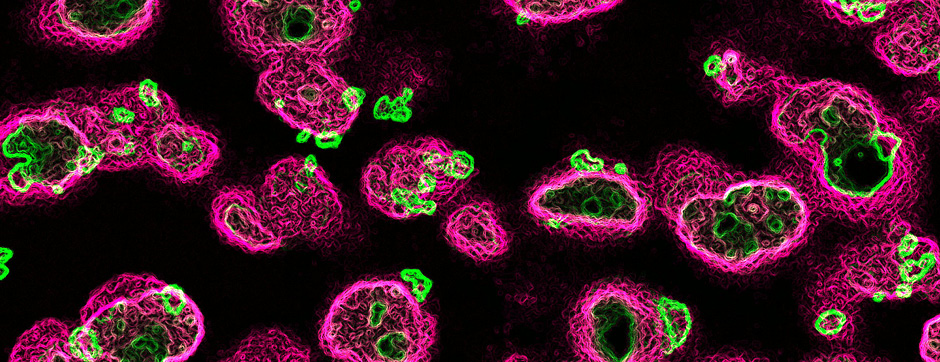
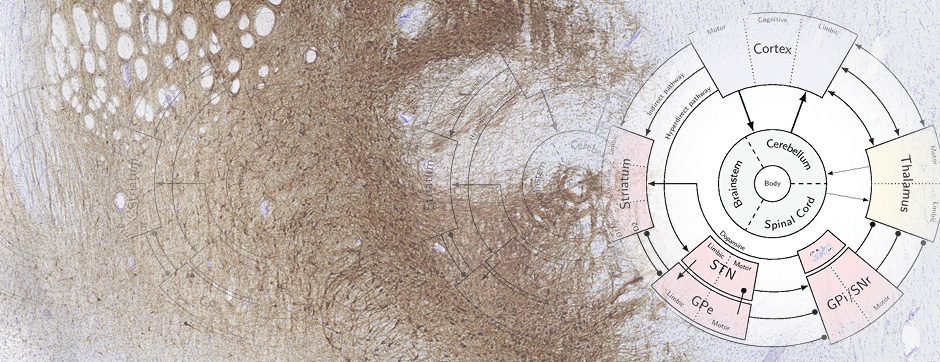
The second part of my PhD was focused on the study of BG dynamics in pathological conditions. In PD, dopaminergic therapy such as the administration of the precursor levodopa reduces the symptomatology and improves movement execution in the early stage of the disease, but quickly triggers abnormal and involuntary movements known as levodopa-induced dyskinesia (LID). LID is very debilitating and refractory to any further drug treatment. Hence, understanding the neuronal mechanism underlying LID is fundamental to develop new therapeutic strategies. Recent studies have shown that LID is caused by excessive neuronal activity in the striatum, however, how such striatal activity impacts BG downstream circuits such as the external globus pallidus (GPe) to generate LID is still unknown. In addition, a subpopulation of GPe neurons called the arkypallidal (Arky-GPe) neurons, directly form a negative feedback loop with the striatum to powerfully control action inhibition in normal conditions. Importantly, the activity and contribution of this GPe action-suppressing pathway during LID is totally unknown.
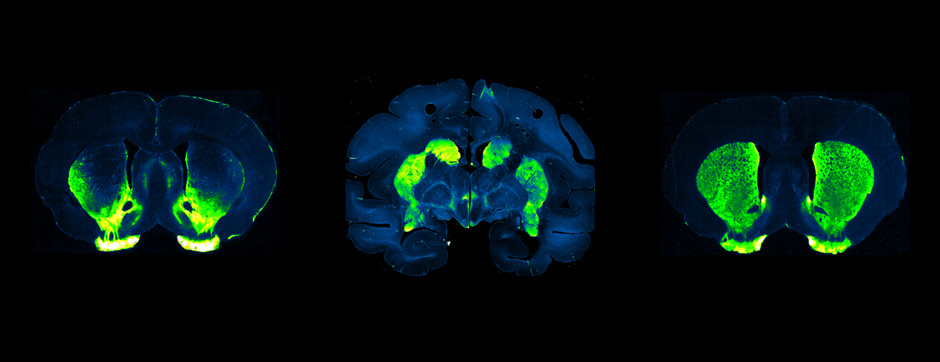
For these reasons, the objective of this project was to characterise the dynamic changes of activity present in Arky-GPe neurons across different disease states, going from healthy condition to the pathophysiological states of PD and LID. In this work, we used optical methods (I.e. fiber photometry and miniscope) to monitor in vivo the calcium activity of Arky-GPe neurons across different motor and disease states. Having characterised the abnormal changes of activity, we then used optogenetic manipulation to test their causal contribution to LID generation. We found that optogenetic reactivation of Arky-GPe neurons during LID reduces hyperkinetic behaviour and promotes normal-like motor behaviour. These results pave the way to understand the complex mechanisms involved in the generation and maintenance of LID.