Senka HADZIBEGOVIC
Behavioural, molecular and electrophysiological characterization of the learning and memory deficits induced in mouse models of Alzheimer's disease
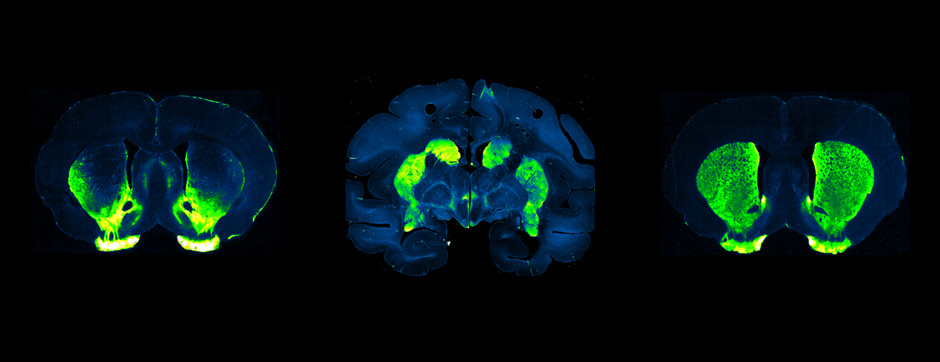
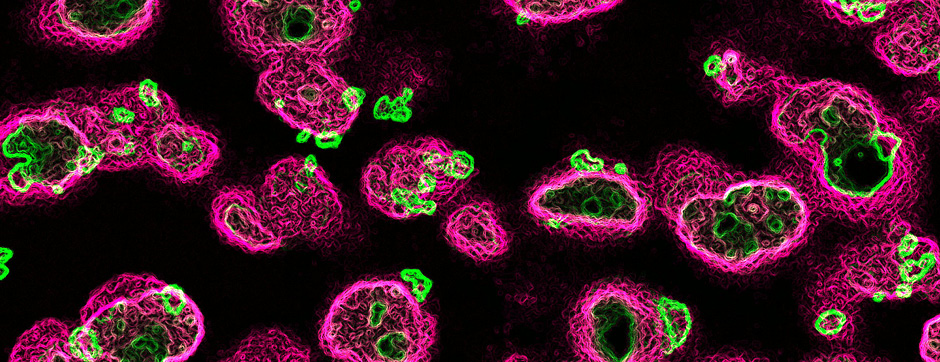
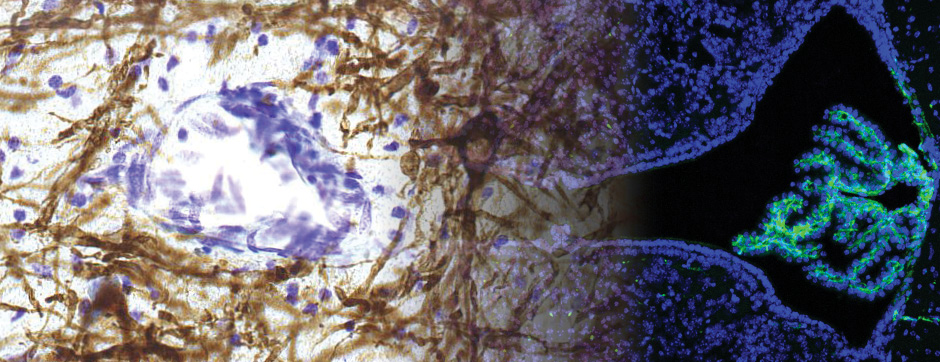
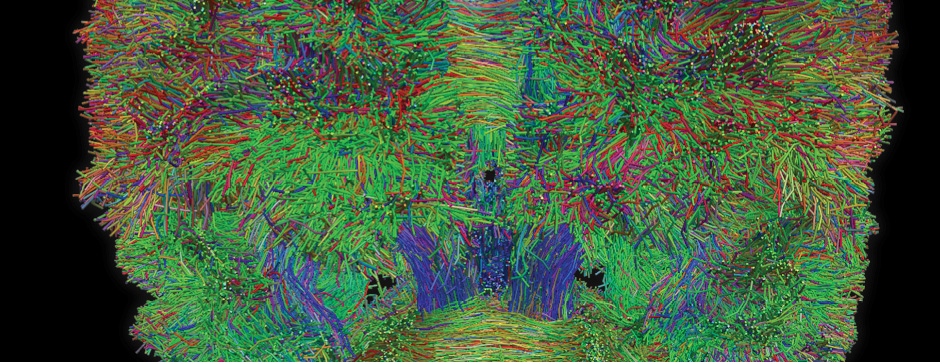
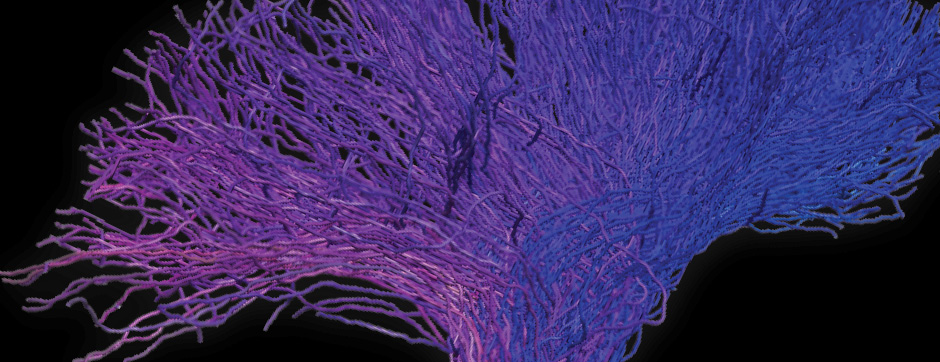
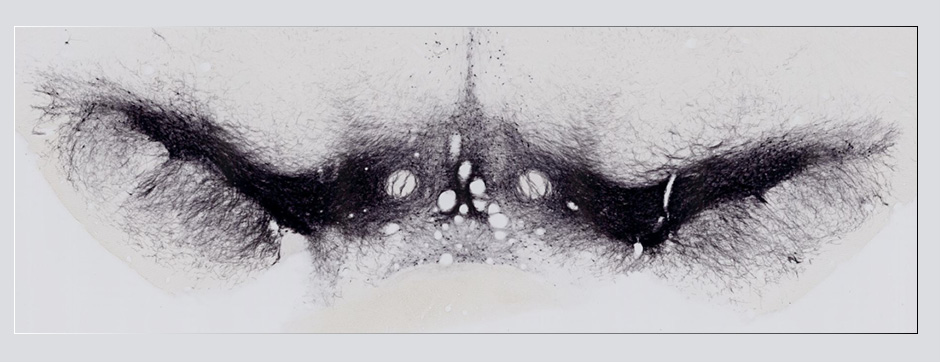
septembre 2015 Directeur(s) de thèse : Bruno BONTEMPI Résumé de thèseCognitive impairments in Alzheimer’s disease (AD) are thought to be related to degenerative synaptic changes caused by the accumulation of amyloid-β peptides (Aβs) in vulnerable brain regions such as the hippocampus. At the molecular level, Aβs bind preferentially to the postsynaptic density of neuronal excitatory synapses, where the scaffolding post-synaptic protein-95 (PSD-95) organizes NMDA receptor (NMDAR) location as well as its downstream signaling. By using an integrative strategy which favoured vertical levels of analyses (from phenotype to molecular events) and combined a set of interrelated correlative and invasive approaches in a double transgenic mouse model of AD (APPswe/PS1dE9 mice), we were successful in establishing that Aβs destabilize the synaptic organization and increases the extrasynaptic pool of GluN2B-containing NMDAR in the hippocampus, a reorganization which translates into impaired memory functions. It is also well-known that hippocampal sharp wave-ripples (SWRs) are crucial for memory formation but surprisingly memory impairments produced by accumulation of soluble Aβos observed in AD seem to spare the SWR dynamics during routine behavior. To unravel a potential effect of Aβos on SWRs in cognitively-challenged animals, we submitted vehicle- and Aβo-injected mice to spatial recognition memory testing. While capable of forming short-term memory, Aβ mice exhibited faster forgetting, suggesting successful encoding but an inability to adequately stabilize and/or retrieve previously acquired information. Without prior cognitive requirements, similar properties of SWRs were observed in both groups. In contrast, when cognitively challenged, the post-encoding and -recognition peaks in SWR occurrence observed in controls were abolished in Aβo mice, indicating impaired hippocampal processing of spatial information. Altogether these results identify two new disruptive mechanisms for the spatial memory deficits associated with AD.