Paul-Arnaud GUERIN
Modélisation et recherche de stratégies expérimentales dans l'atrophie multisystématisée.
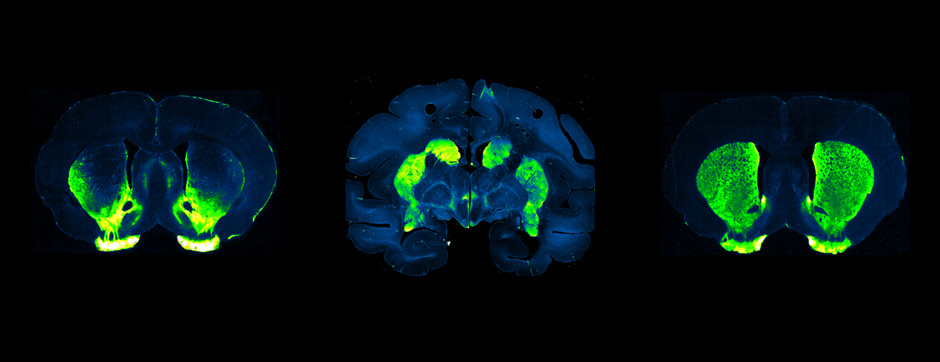
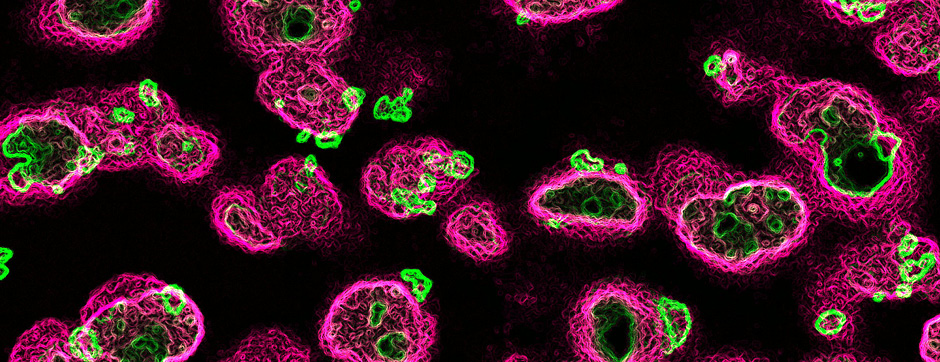
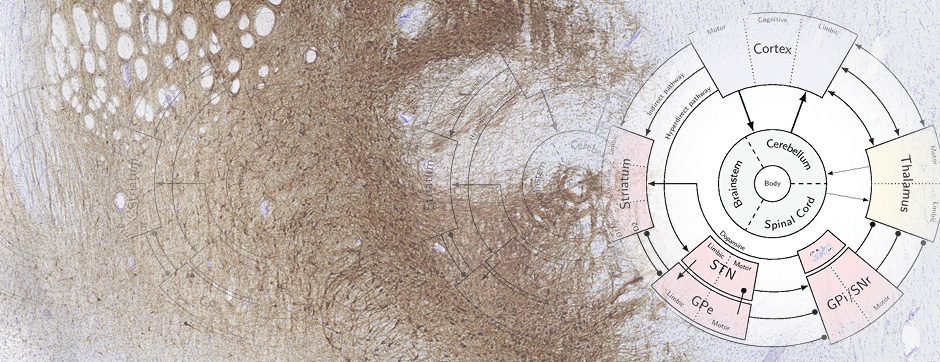
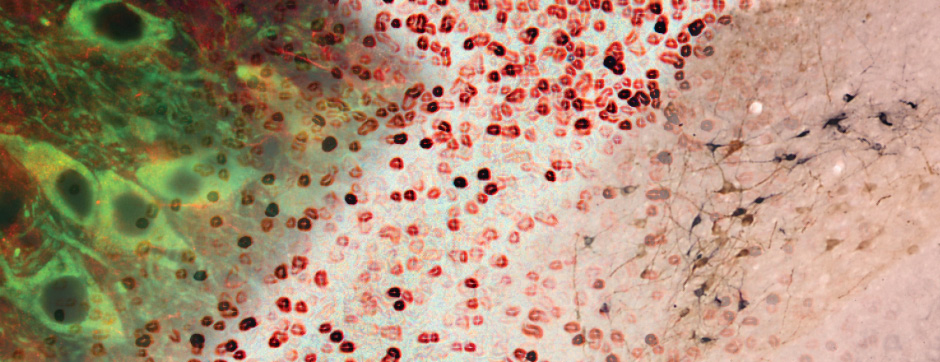
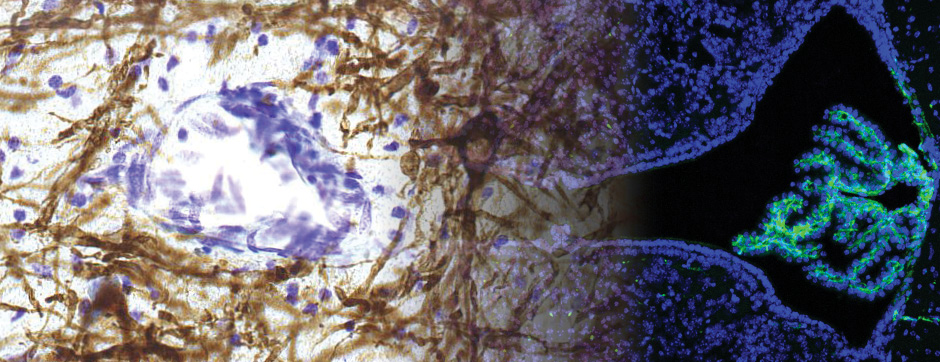
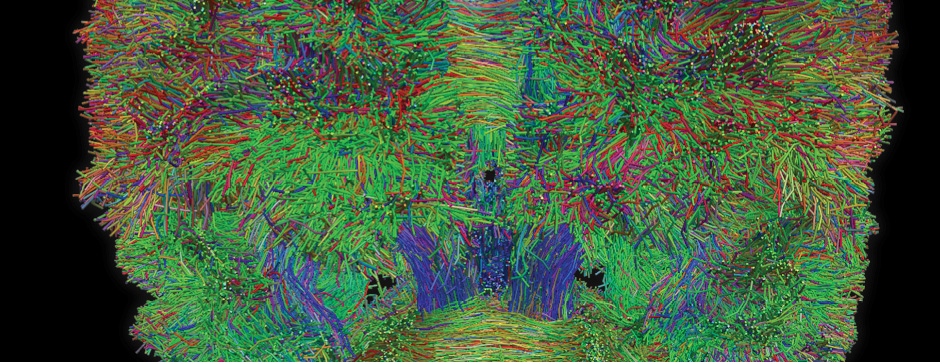
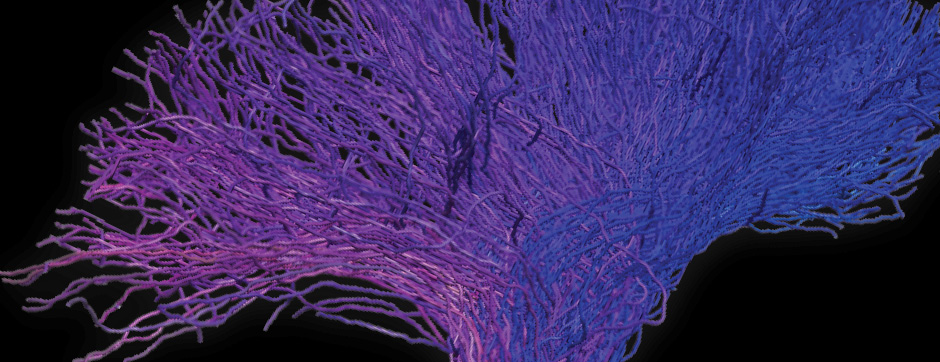
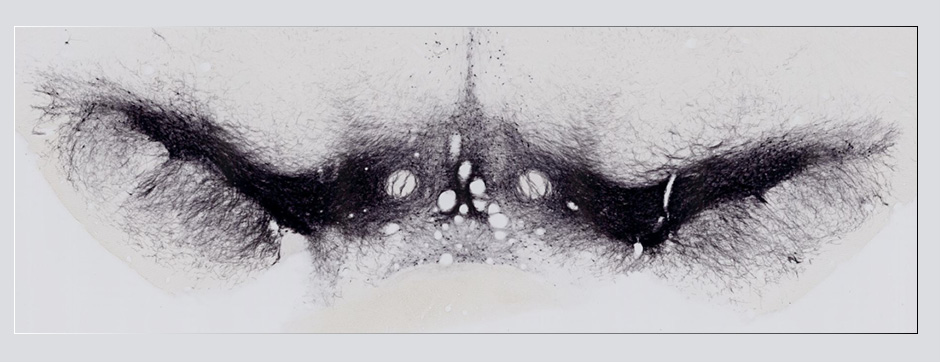
septembre 2018 Directeur(s) de thèse : Wassilios MESSNER Résumé de thèseMultiple system atrophy (MSA) is a rapidly progressing orphan disease characterized by neurodegeneration in several brain regions, including olivopontocerebellar and striatonigral systems, together with several brainstem autonomic nuclei. The hallmark of MSA is the presence of oligodendroglial aggregates named glial cytoplasmic inclusions, which are mostly composed of the protein α-synuclein (α-syn). The neurodegenerative process causes a variable combination of parkinsonism, cerebellar impairment and autonomic dysfunction. No disease modifying therapies, nor peripheral biomarkers that would allow detecting or monitoring the evolution of MSA, are yet available. My PhD work was a multifactorial approach which allowed me to work on the different levels of therapeutic research, from animal modelling to preclinical research, and finally the search for fluid biomarkers in patient samples. We first created new models of MSA based on viral-mediated overexpression of α-syn in striatal oligodendrocytes in rats and non-human primates.We showed in our rat model progressive motor dysfunction, degeneration of dopaminergic neurons of the substantia nigra pars compacta (SNc) and striatal neurons, as well as pathological aggregation of α-syn. For the primate model, we established the specificity of oligodendroglial α-syn expression and the reach of the viral infection. In the second part, we studied the effect of three therapeutic strategies on neurodegeneration and α-syn aggregation in a transgenic murine model of MSA. Rapamycine, known to activate protein degradation through autophagy, showed a partial neuroprotective effect on dopaminergic neurons of the SNc, while intraperitoneal administration of nilotinib, which exerted neuroprotective and anti-aggregative effects in several models of Parkinson’s disease, failed to show any effect in transgenic MSA mice. The last therapeutic strategy aimed to act on brain insulin resistance, which is one of the pathological features found in MSA patient brains, through viral-mediated overexpression of a micro RNA that reduces the expression of the G protein (heterotrimeric guanine nucleotide–binding protein)–coupled receptor kinase 2 (GRK2). The inhibition of the GRK2 kinase, which is involved in mediating insulin resistance, showed neuroprotective effects in the SNc in transgenic MSA mice. The third project of my PhD work consisted in the assessment of several potential fluid biomarkers in MSA patients using the SIMOA technology.