Eléonore Bertin
Étude de l'augmentation du trafic en surface des récepteurs P2X4 de l’ATP à l’aide de nouveaux modèles murins transgéniques : Implications dans les processus mnésiques et la sclérose latérale amyotrophique.
décembre 2019 Directeur(s) de thèse : Eric Boué-Grabot Résumé de thèseATP signaling and surface P2X4 ATP-gated receptor channels are upregulated in various neurological disorders including amyotrophic lateral sclerosis (ALS), a fatal motoneuron (MN) disease characterized by protein misfolding and aggregation leading to cellular degeneration. P2X4 displays a widespread distribution in the central nervous system (CNS) neurons and glial cells as well as in multiple peripheral cell types throughout the body. A key question regarding the role of purinergic signaling in health and disease is the function of this upregulated surface P2X4 state observed in specific cell types.
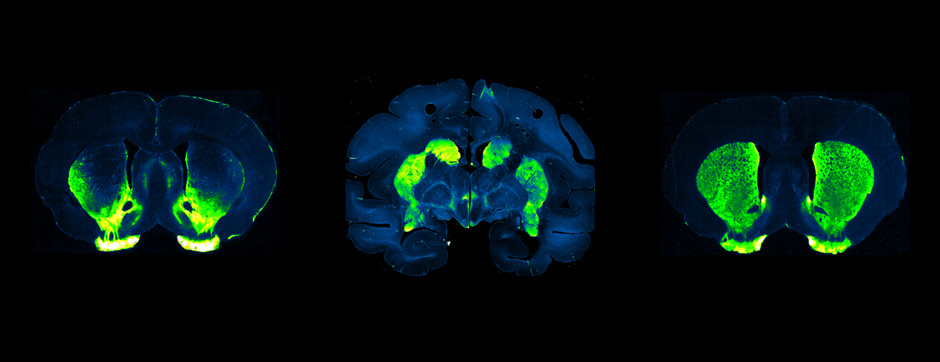
To elucidate the cell-specific functions of P2X4 in a pathological context, a conditional transgenic knock-in P2X4 mouse line (floxed P2X4mCherryIN) was created allowing the Cre activity-dependent genetic swapping of the internalization motif of P2X4 by the fluorescent protein mCherry to prevent constitutive endocytosis of P2X4. We describe and characterize two distinct knock-in mouse lines expressing non-internalized P2X4mCherryIN either in excitatory forebrain neurons (CamK2) or in all cells natively expressing P2X4 (CMV). The genetic substitution of wild-type P2X4 by non-internalized P2X4mCherryIN in both knock-in mouse models does not alter the sparse distribution and subcellular localization of P2X4 but leads to a cell-specific increased surface P2X4 expression mimicking the pathological upregulated P2X4 state. We provide evidence that the increase in P2X4 at the surface of excitatory neurons decreases anxiety and impairs memory processing due to alteration of synaptic plasticity in the hippocampal CA1 region.
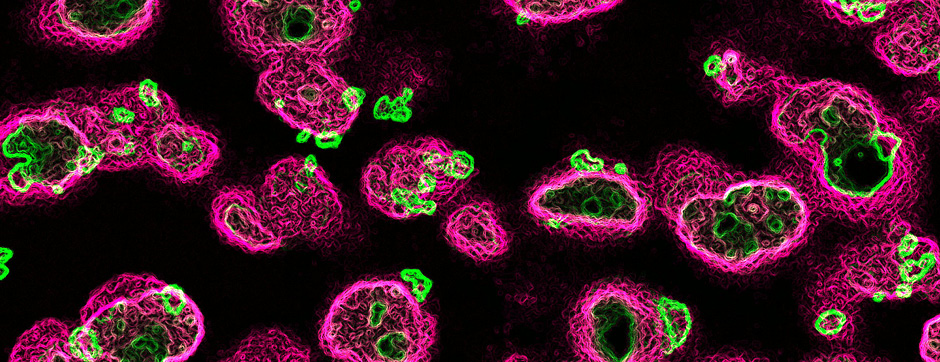
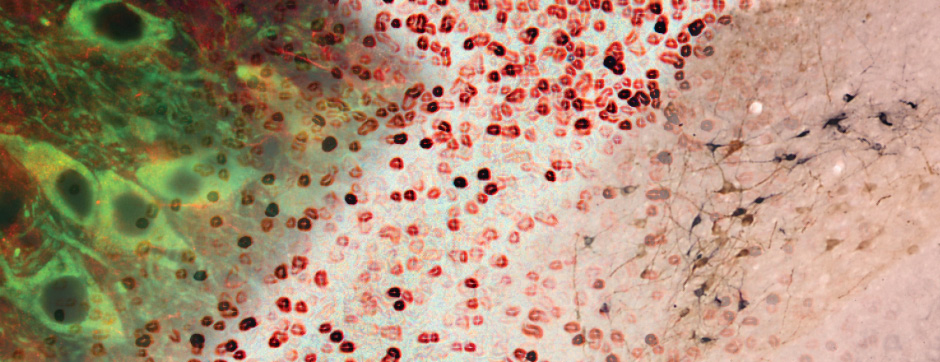
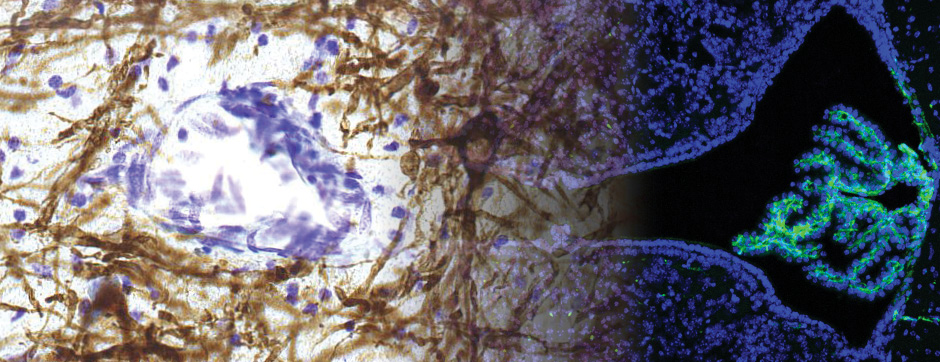
To unravel the implication of P2X4 in ALS pathogenesis, we generate innovating double transgenic mice called SOD1:P2X4KI and SOD1:P2X4KO using the new knock-in CMV mice model expressing the upregulated P2X4 receptor in all cells that expressed natively the P2X4 receptor (P2X4KI) or a trangenic mice lacking the P2X4 gene (P2X4KO) with most commonly used ALS model carrying the human SOD1-G93A mutation (SOD1). Interestingly, the ablation of the P2X4 gene as well as the expression of non-internalized P2X4 in SOD1 mice have a significant and positive impact on motor performances and animal survival revealing that P2X4 are active and complex players in ALS progression. In SOD1 mice spinal cord, the expression of P2X4 is initially restricted to MN and increased in microglia during the symptomatic phase of ALS, and P2X4 has a dual role on inflammation markers expression during the progression of the disease. In parallel, P2X4 surface expression significantly increased in peritoneal macrophages of SOD1 mice during ALS progression even from the presymptomatic stages suggesting that P2X4 may represent an early biomarker of ALS. Moreover, we reveal that the mechanism underlying the surface upregulation of P2X4 receptor in ALS models over the time can be explained by a competitive and progressive alteration of P2X4 constitutive internalization by SOD1 misfolded protein leading to MN death and associated neuroinflammation.
Overall, we provide an innovative knock-in P2X4 model to study the functional contributions of upregulated P2X4 receptor in specific cells of the nervous system but also in peripheral tissues throughout the body that will be helpful for study many others pathologies besides ALS.
Keywords : Transgenic mice – P2X4 – ALS