Équipe 3
Équipe 3
Dopamine and neuronal assemblies
LADEVEZE Elodie (Engineer ) LE BON-JEGO Morgane (MCU) GLANGETAS Christelle (Post-doctoral researcher) GARCIA BAOS Alba (Post-doctoral researcher) GUILLAUMIN Adriane (Post-doctoral researcher) SIERRA Teresa (Post-doctoral researcher) BONAMY Léa (Phd Student) DE LAS HERRAS-GARCIA Laura (PhD Student ) PERROT Emma (PhD Student ) SCHARNHOLZ Jakob (PhD Student) VILLACANA-MUNOZ Viviana (PhD Student )
For any further information, please contact
Dr Jérôme BAUFRETON
jerome.baufreton@u-bordeaux.fr
Dr François GEORGES
Research subject
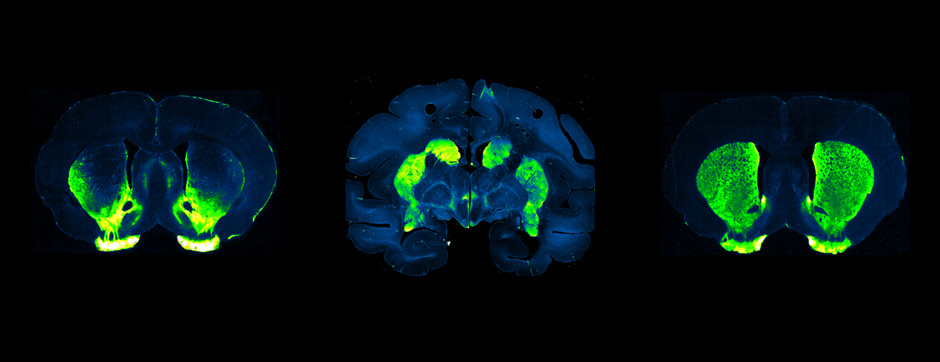
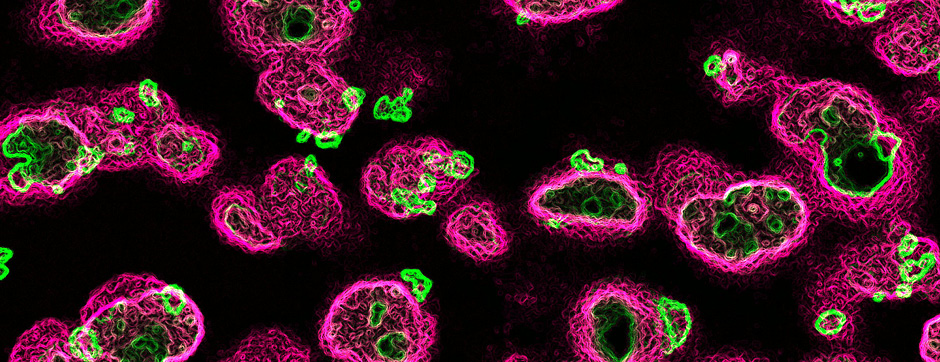
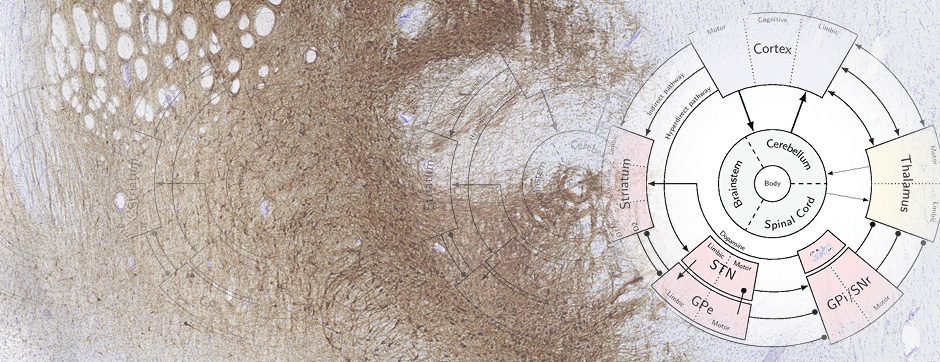
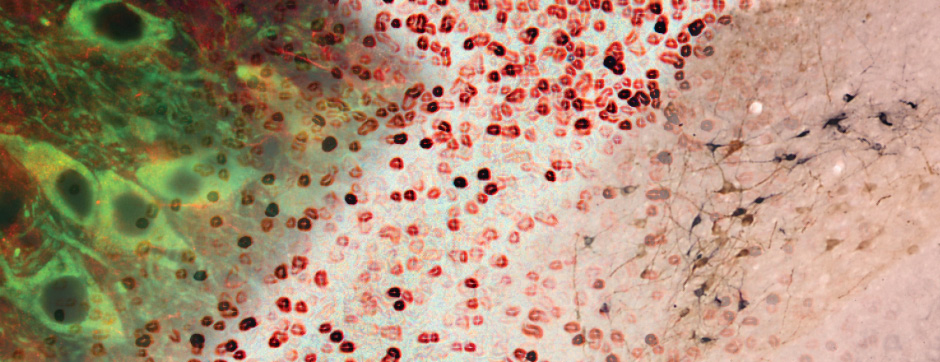
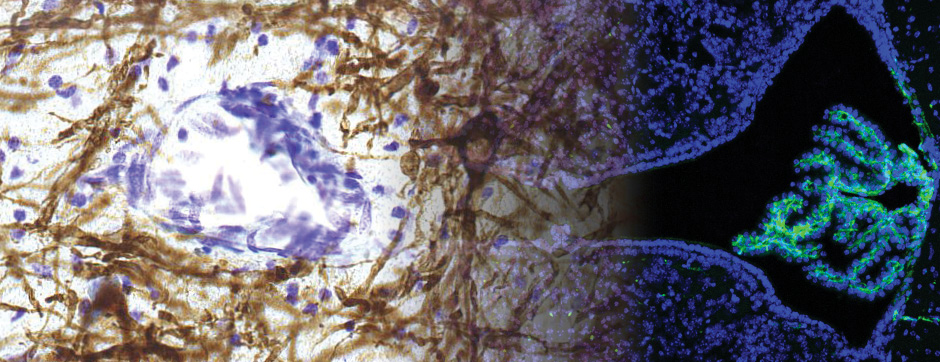
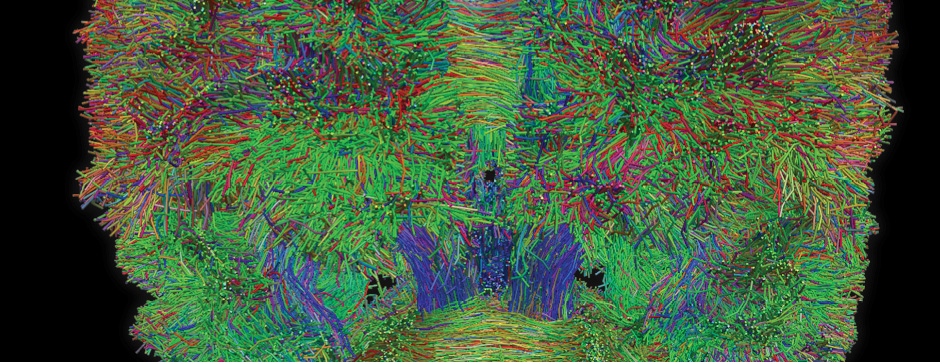
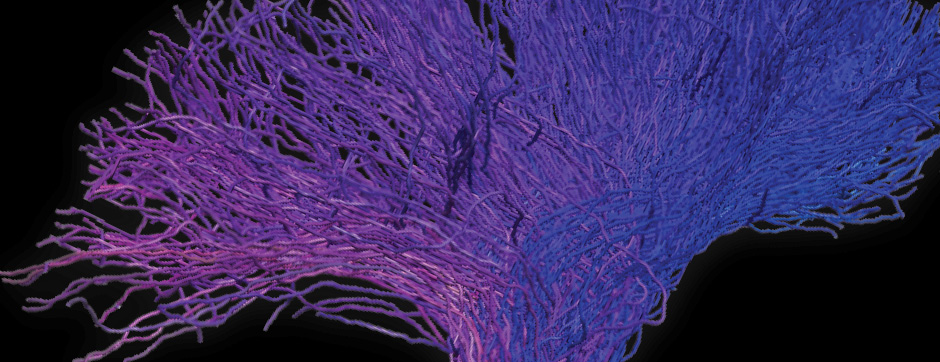
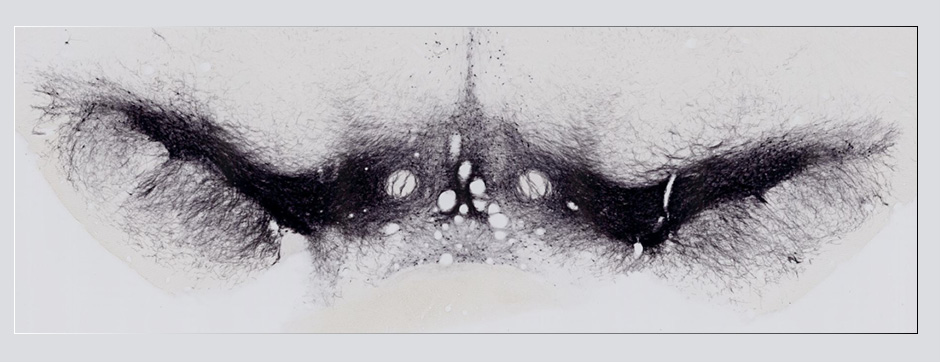
Motion and emotion are intimately linked. The major goal of our research is to delineate the functions of dopamine in the « extended basal ganglia network » (EBG). EBG is a neural circuit controlling movement and emotional states (voluntary movement, associative learning, explorative behavior using processing of relevant sensory information, stress), but is also involved in neurological and psychiatric disorders (Parkinson’s disease, Huntington’s disease, anxiety). The objective of our team is to establish a base of knowledge to define therapeutic avenues to treat the symptoms of neurological and psychiatric diseases related to the dopaminergic system. We are particularly interested in the control of synaptic transmission and plasticity by dopamine.
We study these mechanisms at the synapse, cell, neuronal circuit and behavioral levels. We combine state-of-the-art technology, such as: in and ex vivo electrophysiology (coupled with cell identification after single cell labeling), intersectional viral approaches to identify (tracing), manipulate (optogenetics, chemogenetics) and record (endoscopic imaging & fiberphotometry) neuronal activity (calcium imaging) or dopamine release (dopamine sensor) in animals engaged in a behavioral task. Using those innovative neurotechniques we aim to uncover how defined subsets of neurons within the EBG encode aspects of motion and emotion.
Dernières publications
Criteria : Author : "Jerome,Baufreton; François,Georges", Publication type : "('ART')"
Number of occurrences founded : 86.
- titre
- GAP43 Located on Corticostriatal Terminals Restrains Novelty-Induced Hyperactivity in Mice
- auteur
- Irene Maroto, Carlos Costas-Insua, Carlos Montero-Fernández, Alba Hermoso-López, Margaux Lebouc, Raquel Bajo-Grañeras, Alicia Álvaro-Blázquez, Cristina Blázquez, Astrid Cannich, Giovanni Marsicano, Ricardo Martín, Jérôme Baufreton, Ignacio Rodríguez-Crespo, Luigi Bellocchio, Manuel Guzmán
- article
- Journal of Neuroscience, 2024, 44 (39), pp.e0701242024. ⟨10.1523/JNEUROSCI.0701-24.2024⟩
- identifiant
- hal-04727363
- titre
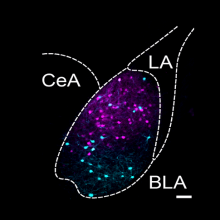
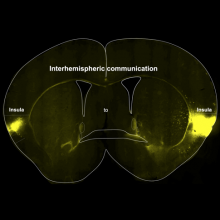
- A population of Insula neurons encodes for social preference only after acute social isolation in mice
- auteur
- Christelle Glangetas, Adriane Guillaumin, Elodie Ladevèze, Anaelle Braine, Manon Gauthier, Léa Bonamy, Evelyne Doudnikoff, Thibault Dhellemmes, Marc Landry, Erwan Bézard, Stephanie Caille, Anne Taupignon, Jérôme Baufreton, François Georges
- article
- Nature Communications, 2024, 15 (1), pp.7142. ⟨10.1038/s41467-024-51389-4⟩
- identifiant
- hal-04681539
- titre
- Nucleus accumbens D1- and D2-expressing neurons control the balance between feeding and activity-mediated energy expenditure
- auteur
- Roman Walle, Anna Petitbon, Giulia R Fois, Christophe Varin, Enrica Montalban, Lola Hardt, Andrea Contini, Maria Florencia Angelo, Mylène Potier, Rodrigue Ortole, Asma Oummadi, Véronique de Smedt-Peyrusse, Roger A Adan, Bruno Giros, Francis Chaouloff, Guillaume Ferreira, Alban de Kerchove D’exaerde, Fabien Ducrocq, François Georges, Pierre Trifilieff
- article
- Nature Communications, 2024, 15 (1), pp.2543. ⟨10.1038/s41467-024-46874-9⟩
- identifiant
- hal-04670379
- titre
- Age-Dependent Modulation of Layer V Pyramidal Neuron Excitability in the Mouse Primary Motor Cortex by D1 Receptor Agonists and Antagonists
- auteur
- Valentin Plateau, Jérôme Baufreton, Morgane Le Bon-Jégo
- article
- Neuroscience, 2024, 536, pp.21-35. ⟨10.1016/j.neuroscience.2023.11.006⟩
- identifiant
- hal-04742029
- titre
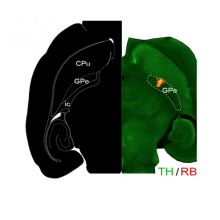
- Spatiomolecular Characterization of Dopamine D2 Receptors Cells in the Mouse External Globus Pallidus
- auteur
- Julie Espallergues, Jihane Boubaker-Vitre, Audrey Mignon, Maelle Avrillon, Morgane Le Bon-Jego, Jerome Baufreton, Emmanuel Valjent
- article
- Current Neuropharmacology, In press, 22 (9), pp.1528-1539. ⟨10.2174/1570159X21666230720121027⟩
- identifiant
- hal-04181821